Embark on a scientific odyssey with the Ions and Isotopes Practice Worksheet, a comprehensive resource designed to illuminate the intricacies of these fundamental chemical concepts. Delve into the captivating realm of ions and isotopes, unraveling their unique properties and exploring their indispensable applications in various fields.
This meticulously crafted worksheet empowers learners with a thorough understanding of the fundamental principles governing ions and isotopes, enabling them to navigate the complexities of chemistry with confidence.
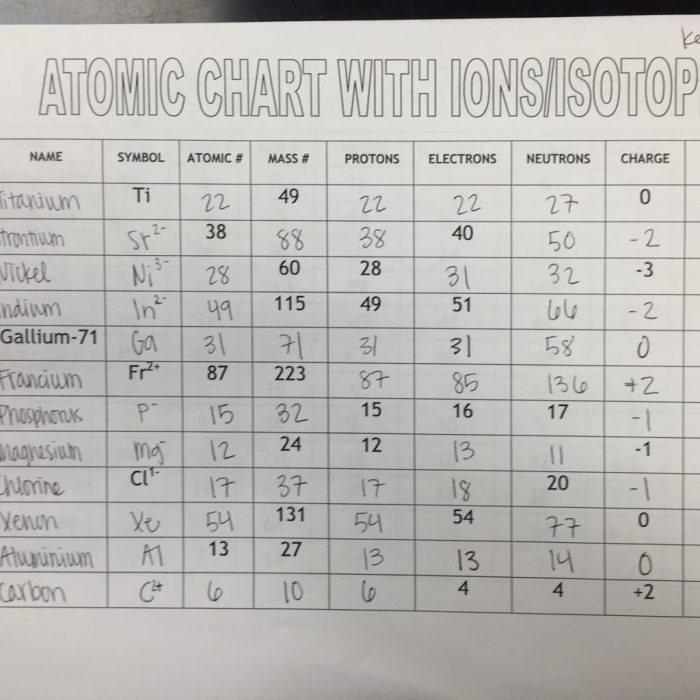
Ions and Isotopes
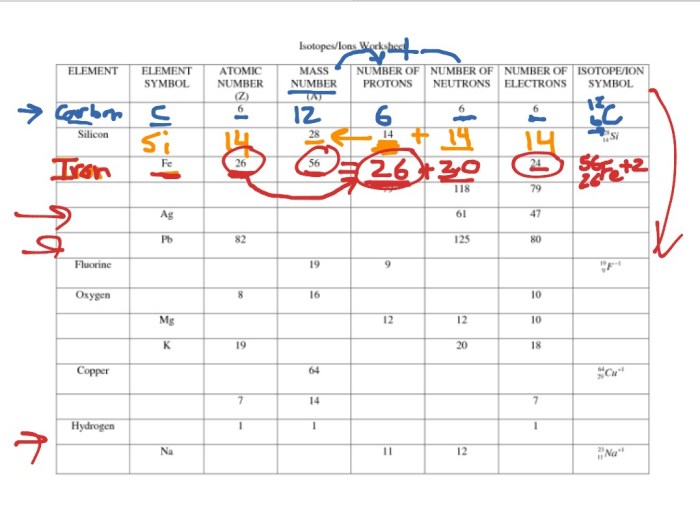
Ions and isotopes are two types of atoms that have different numbers of electrons or neutrons, respectively. Ions are atoms that have gained or lost electrons, while isotopes are atoms of the same element that have different numbers of neutrons.
The difference between ions and isotopes is that ions have a different number of electrons than protons, while isotopes have the same number of electrons and protons but a different number of neutrons.
Properties of Ions
Ions are formed when an atom gains or loses electrons. When an atom gains electrons, it becomes a negative ion. When an atom loses electrons, it becomes a positive ion. The charge of an ion is determined by the number of electrons that it has gained or lost.
Ions are attracted to each other by electrostatic forces. The strength of the attraction between two ions is proportional to the charges of the ions and inversely proportional to the distance between them.
Ions are found in many different compounds, including salts, acids, and bases. Ions are also found in the human body, where they play an important role in many different processes, such as nerve conduction and muscle contraction.
Properties of Isotopes, Ions and isotopes practice worksheet
Isotopes are atoms of the same element that have different numbers of neutrons. The number of protons in an atom determines the element to which it belongs. The number of neutrons in an atom does not affect the element to which it belongs, but it can affect the isotope’s properties.
Isotopes are formed when an atom undergoes nuclear reactions. Nuclear reactions can occur naturally or they can be induced artificially.
Isotopes are found in many different materials, including rocks, minerals, and plants. Isotopes are also found in the human body, where they play an important role in many different processes, such as metabolism and DNA replication.
Applications of Ions and Isotopes
Ions and isotopes have a wide variety of applications in science, medicine, and industry.
- Ions are used in batteries, fuel cells, and other electrochemical devices.
- Ions are also used in water treatment, food processing, and other industrial processes.
- Isotopes are used in nuclear power plants, medical imaging, and other scientific research.
- Isotopes are also used in dating archaeological artifacts and geological formations.
Frequently Asked Questions: Ions And Isotopes Practice Worksheet
What are the key differences between ions and isotopes?
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge. Isotopes, on the other hand, are atoms of the same element that have different numbers of neutrons, leading to variations in atomic mass but no change in chemical properties.
How are ions formed?
Ions are formed when an atom gains or loses electrons through processes such as ionization (loss of electrons) or electron capture (gain of electrons).
What are some examples of applications of isotopes?
Isotopes have a wide range of applications, including medical imaging (e.g., radioactive iodine in thyroid scans), nuclear power generation (e.g., uranium-235), and geological dating (e.g., carbon-14 dating).