Give the IUPAC Name for Each Compound: A Comprehensive Guide to Chemical Nomenclature provides a comprehensive overview of the International Union of Pure and Applied Chemistry (IUPAC) guidelines for naming organic compounds. This guide will equip you with the knowledge and skills necessary to confidently and accurately assign IUPAC names to a wide range of organic molecules.
IUPAC nomenclature is a systematic and standardized system for naming organic compounds. It ensures that compounds are assigned unique and unambiguous names that accurately reflect their structure and composition. This guide will cover the fundamental principles of IUPAC nomenclature, including the rules for naming alkanes, alkenes, alkynes, cyclic compounds, branched compounds, and compounds containing functional groups.
Nomenclature Rules
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of guidelines for naming organic compounds. These rules aim to provide a systematic and unambiguous way to identify and describe organic molecules.
The IUPAC nomenclature system is based on the following principles:
- The base part of the name reflects the parent hydrocarbon chain.
- Prefixes and suffixes are used to indicate the presence of functional groups.
- Locants are used to specify the position of substituents on the parent chain.
Functional Groups
The following table provides a summary of the prefixes and suffixes used to indicate the presence of common functional groups:
| Functional Group | Prefix | Suffix |
|---|---|---|
| Alkyl | alkyl- | -ane |
| Alkenyl | alkenyl- | -ene |
| Alkynyl | alkynyl- | -yne |
| Alcohol | hydroxy- | -ol |
| Aldehyde | oxo- | -al |
| Ketone | oxo- | -one |
| Carboxylic acid | carboxy- | -oic acid |
| Ester | alkoxycarbonyl- | -oate |
| Amine | amino- | -amine |
| Nitrile | cyano- | -nitrile |
Locants
Locants are used to specify the position of substituents on the parent chain. The locant is placed before the name of the substituent and indicates the carbon atom to which the substituent is attached.
For example, the compound 2-propanol has a hydroxyl group attached to the second carbon atom of the propane chain.
Examples
Here are some examples of IUPAC names for organic compounds:
- CH 3CH 2OH: Ethanol
- CH 3CH=CHCH 3: 2-Butene
- CH 3COCH 3: Propanone
- CH 3CH 2COOH: Propanoic acid
- CH 3CH 2CH 2NH 2: Propylamine
Functional Groups
Functional groups are specific groups of atoms within a molecule that determine its chemical properties and reactivity. They are named according to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules.
Common Functional Groups
Here is a table of common functional groups and their IUPAC names:
| Functional Group | IUPAC Name |
|---|---|
| Hydroxyl | -ol |
| Carbonyl | -one |
| Carboxyl | -oic acid |
| Amine | -amine |
| Alkyl halide | -yl halide |
The presence of functional groups affects the naming of compounds by providing the root name. For example, a compound with a hydroxyl group is named as an alcohol, while a compound with a carbonyl group is named as a ketone.
Alkanes
Alkanes are acyclic hydrocarbons that contain only carbon and hydrogen atoms. They are characterized by a single bond between each carbon atom and its neighboring carbon atoms, forming a chain or branched structure. The general formula for alkanes is CnH2n+2, where n represents the number of carbon atoms in the molecule.
Structure and Naming of Alkanes
Alkanes have a tetrahedral geometry around each carbon atom. The carbon-carbon bonds are arranged in a staggered conformation, which minimizes steric hindrance. The naming of alkanes follows the IUPAC (International Union of Pure and Applied Chemistry) nomenclature system. The root name of an alkane is based on the number of carbon atoms in the chain.
The suffix “-ane” is added to indicate that the compound is an alkane.
Prefixes for Number of Carbon Atoms
The prefixes used to indicate the number of carbon atoms in alkanes are:
- Meth- (1 carbon)
- Eth- (2 carbons)
- Prop- (3 carbons)
- But- (4 carbons)
- Pent- (5 carbons)
- Hex- (6 carbons)
- Hept- (7 carbons)
- Oct- (8 carbons)
- Non- (9 carbons)
- Dec- (10 carbons)
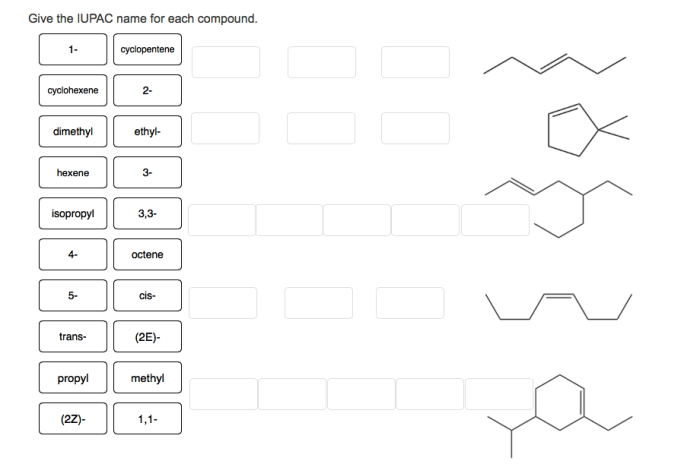
Alkenes
Alkenes are a class of hydrocarbons that contain at least one carbon-carbon double bond. They are unsaturated hydrocarbons, meaning they have fewer hydrogen atoms than the corresponding alkane. The general formula for an alkene is CnH2n, where n is the number of carbon atoms in the molecule.The
IUPAC name for an alkene is based on the number of carbon atoms in the parent chain and the location of the double bond. The parent chain is the longest chain of carbon atoms that contains the double bond. The suffix “-ene” is used to indicate the presence of a double bond.
The location of the double bond is indicated by a number, which is placed before the suffix. The number corresponds to the carbon atom at which the double bond begins.For example, the IUPAC name for the alkene with three carbon atoms and a double bond between the first and second carbon atoms is propene.
The parent chain is propane, and the double bond is located between the first and second carbon atoms.Here is a table of common alkenes and their IUPAC names:| Alkene | IUPAC Name ||—|—|| CH2=CH2 | Ethene || CH3CH=CH2 | Propene || CH3CH2CH=CH2 | Butene || CH3CH2CH2CH=CH2 | Pentene || CH3CH2CH2CH2CH=CH2 | Hexene |
Alkynes
Alkynes are hydrocarbons that contain at least one carbon-carbon triple bond. They are unsaturated hydrocarbons, meaning they have fewer hydrogen atoms than the corresponding alkane with the same number of carbon atoms.
IUPAC Nomenclature of Alkynes, Give the iupac name for each compound
The IUPAC name of an alkyne is derived from the name of the parent alkane by replacing the -ane suffix with the -yne suffix. The location of the triple bond is indicated by a number. For example, the IUPAC name of the alkyne with a triple bond between the first and second carbon atoms is 1-butyne.
Common Alkynes and their IUPAC Names
| IUPAC Name | Common Name |
|---|---|
| Ethyne | Acetylene |
| 1-Butyne | Butylacetylene |
| 2-Butyne | Dimethylacetylene |
| 1-Pentyne | Pentylacetylene |
| 2-Pentyne | Methylbutylacetyle |
Use of Suffixes to Indicate the Presence of Triple Bonds in Alkynes
The presence of a triple bond in an alkyne is indicated by the suffix -yne. The number of triple bonds in an alkyne is indicated by the number of -yne suffixes. For example, a hydrocarbon with two triple bonds is named as a diyne, a hydrocarbon with three triple bonds is named as a triyne, and so on.
Cyclic Compounds
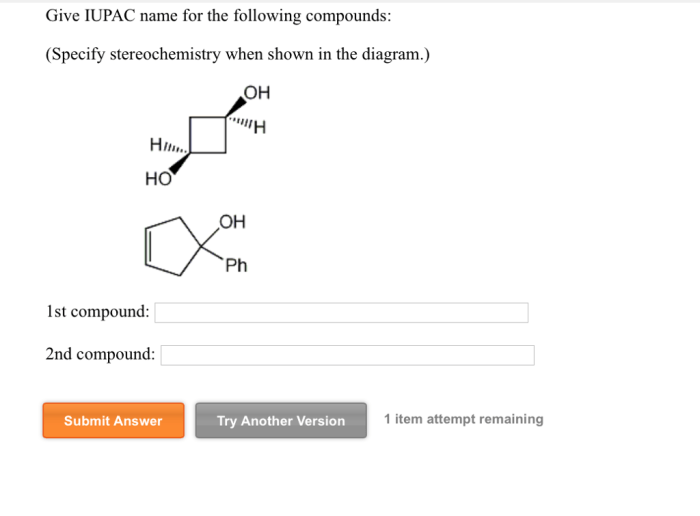
Cyclic compounds are organic compounds that contain one or more rings of atoms. The rings can be composed of carbon atoms only, or they can contain other atoms, such as nitrogen, oxygen, or sulfur.
Nomenclature of Cyclic Compounds
The IUPAC nomenclature system for cyclic compounds is based on the following rules:
- The base name of the compound is determined by the number of carbon atoms in the ring.
- The prefixes “cyclo” and “cycloalk” are used to indicate that the compound is cyclic.
- The suffix “-ane” is used for saturated cyclic compounds, while the suffix “-ene” is used for unsaturated cyclic compounds.
- The location of substituents on the ring is indicated by numbers.
The following table lists some common cyclic compounds and their IUPAC names:
| Formula | Name |
|---|---|
| C3H6 | Cyclopropane |
| C4H8 | Cyclobutane |
| C5H10 | Cyclopentane |
| C6H12 | Cyclohexane |
| C7H14 | Cycloheptane |
The prefixes “cyclo” and “cycloalk” are used to indicate the number of carbon atoms in the ring. For example, the prefix “cycloprop” indicates that the ring contains three carbon atoms, while the prefix “cyclohex” indicates that the ring contains six carbon atoms.
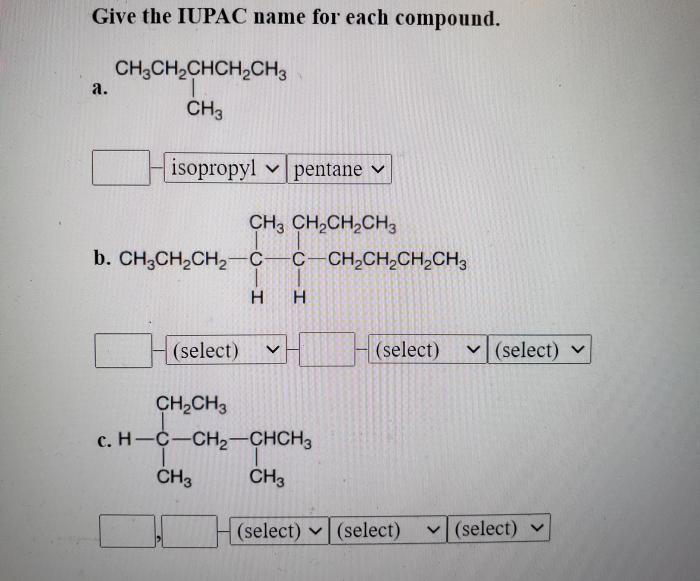
Branched Compounds
Branched compounds are alkanes, alkenes, or alkynes that have one or more alkyl groups attached to the main carbon chain. The alkyl groups are named as substituents, and their position on the main chain is indicated by a number.
Naming Branched Alkanes
To name a branched alkane, follow these steps:
- Identify the longest carbon chain in the molecule. This is the parent chain.
- Number the carbon atoms in the parent chain from one end to the other.
- Identify the alkyl groups attached to the parent chain. These are the substituents.
- Name the substituents using the prefixes methyl, ethyl, propyl, butyl, and so on.
- Indicate the position of the substituents on the parent chain by a number. This number corresponds to the carbon atom to which the substituent is attached.
- Combine the names of the substituents and the parent chain to form the name of the branched alkane.
For example, the branched alkane shown below is named 2-methylbutane.
Naming Branched Alkenes
To name a branched alkene, follow the same steps as for naming a branched alkane, with the following modifications:
- The suffix -aneis replaced by -ene.
- The position of the double bond is indicated by a number. This number corresponds to the carbon atom at one end of the double bond.
For example, the branched alkene shown below is named 2-methyl-2-butene.
Naming Branched Alkynes
To name a branched alkyne, follow the same steps as for naming a branched alkene, with the following modifications:
- The suffix -eneis replaced by -yne.
- The position of the triple bond is indicated by a number. This number corresponds to the carbon atom at one end of the triple bond.
For example, the branched alkyne shown below is named 2-methyl-3-hexyne.
Functional Group Priority: Give The Iupac Name For Each Compound
When naming compounds, it is important to consider the priority of functional groups. The priority of a functional group determines which part of the compound is considered the parent chain and which part is considered the substituent. The rules for determining the priority of functional groups are as follows:
- Carboxylic acids have the highest priority.
- Aldehydes and ketones have the second highest priority.
- Alcohols, ethers, and amines have the third highest priority.
- Alkenes and alkynes have the fourth highest priority.
- Alkanes have the lowest priority.
The following table lists the functional groups and their priority order:
| Functional Group | Priority |
|---|---|
| Carboxylic acid | 1 |
| Aldehyde | 2 |
| Ketone | 2 |
| Alcohol | 3 |
| Ether | 3 |
| Amine | 3 |
| Alkene | 4 |
| Alkyne | 4 |
| Alkane | 5 |
The priority of the functional group affects the naming of the compound. The parent chain is the longest chain of carbon atoms that contains the highest priority functional group. The substituents are the atoms or groups of atoms that are attached to the parent chain.
The substituents are named according to their priority, with the highest priority substituent being named first.
Examples and Exercises
To solidify understanding of IUPAC nomenclature, it is essential to practice naming compounds and identifying their structures based on their IUPAC names. The following examples and exercises provide opportunities to refine these skills.
Examples of Compounds and their IUPAC Names
- CH 3CH 2CH 2CH 2CH 3: Pentane
- CH 3CH=CHCH 2CH 3: 1-Pentene
- CH 3C≡CCH 2CH 3: 1-Pentyne
- CH 3CH 2CH(CH 3)CH 2CH 3: 2-Methylpentane
- CH 3CH 2CH 2CH(CH 3) 2: 3-Methylpentane
- CH 3CH 2CH(CH 3)CH(CH 3)CH 2CH 3: 2,3-Dimethylhexane
- CH 3CH 2CH=CHCH 2CH 3: 1-Hexene
- CH 3CH 2CH 2C≡CCH 2CH 3: 1-Hexyne
- CH 3CH 2CH(CH 3)CH=CH 2: 4-Methyl-1-pentene
- CH 3CH 2CH 2CH(CH 3)CH 2CH 2CH 3: 3-Ethylhexane
Exercises for IUPAC Naming
- Name the following compound: CH3CH 2CH(CH 3)CH 2CH 2CH 2CH 3
- Draw the structure of 2,2-dimethyl-3-hexene.
- Name the following compound: CH 3CH 2C≡CCH(CH 3)CH 2CH 3
- Draw the structure of 4-ethyl-2-methyl-1-heptyne.
- Name the following compound: (CH 3) 3CCH 2CH 2CH=CH 2
Common Errors in IUPAC Naming
To avoid errors in IUPAC naming, it is important to pay attention to the following:
- Identify the parent chain correctly, which is the longest continuous chain of carbon atoms.
- Number the parent chain to give the lowest numbers to the substituents.
- Use the correct prefixes for the substituents (e.g., methyl, ethyl, propyl).
- Use the correct prefixes for the degree of unsaturation (e.g., -ene, -yne).
- Use hyphens to separate numbers and prefixes from the parent chain name.
- Use commas to separate multiple substituents.
User Queries
What is IUPAC nomenclature?
IUPAC nomenclature is a systematic and standardized system for naming organic compounds developed by the International Union of Pure and Applied Chemistry (IUPAC).
Why is IUPAC nomenclature important?
IUPAC nomenclature ensures that organic compounds are assigned unique and unambiguous names that accurately reflect their structure and composition. This facilitates clear and precise communication among chemists and other scientists.
How do I use IUPAC nomenclature to name a compound?
To use IUPAC nomenclature to name a compound, you need to identify the parent chain, functional groups, and substituents present in the molecule. The parent chain is the longest continuous chain of carbon atoms in the molecule. Functional groups are atoms or groups of atoms that have characteristic chemical properties.
Substituents are atoms or groups of atoms that are attached to the parent chain.